Information for Researchers
RADIANT is committed to maximizing its contribution to the greater scientific community. Beyond published findings, RADIANT may also offer researchers everything from methods and technologies developed on study to the actual data and samples collected from participants.
Data & Samples Collected in RADIANT
A variety of different data types are collected as a part of RADIANT, including clinical data, genetics and metabolomics data, and laboratory data. The RADIANT Data Coordinating Center (DCC) manages, curates, integrates, and provisions these data assets for analysis by RADIANT and approved collaborators. Below is a summary of the different data types and samples collected throughout the study.
Clinical Data:
- Autoantibodies
- ASA24
- Birth History
- Demographics
- Diabetes History
- Family History
- Environmental Exposures
- Medications
- Medical History
- Physical Assessment
- PROMIS
'Omics Analytes:
- Mitochondrial DNA Sequencing
- Metabolomics
- RNA Sequencing
- Sanger Sequencing
- Whole Genome Sequencing
Laboratory Data:
- Islet Autoantibody Information
- Fasting Glucose and C-peptide
- Lipid Panel
- Comprehensive Metabolic Panel
- HbA1c
- OGTT
Stored Samples:
- Serum aliquots
- Plasma aliquots
- DNA aliquots
- RNA aliquots
- Urine aliquots
- Peripheral blood monocytes
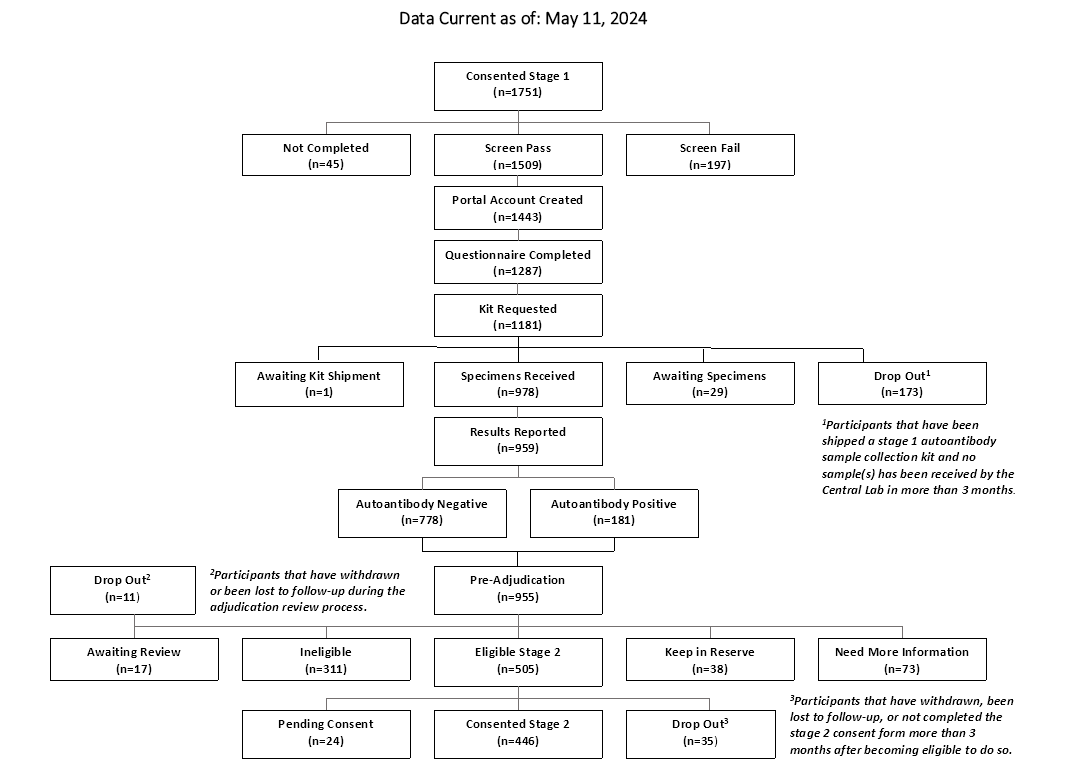
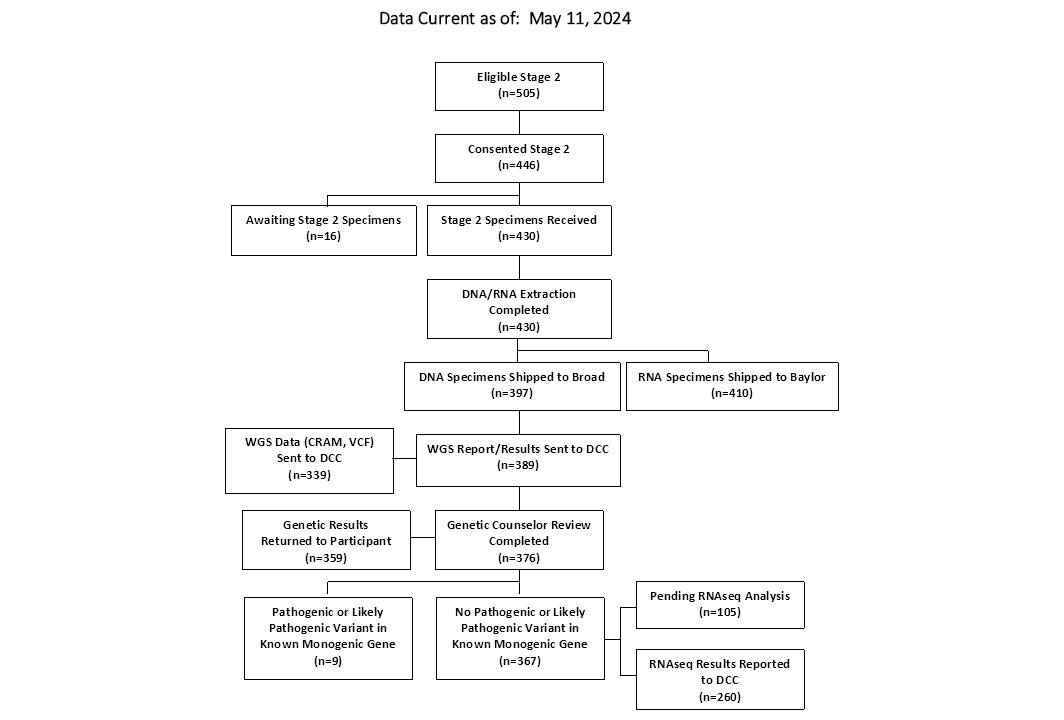
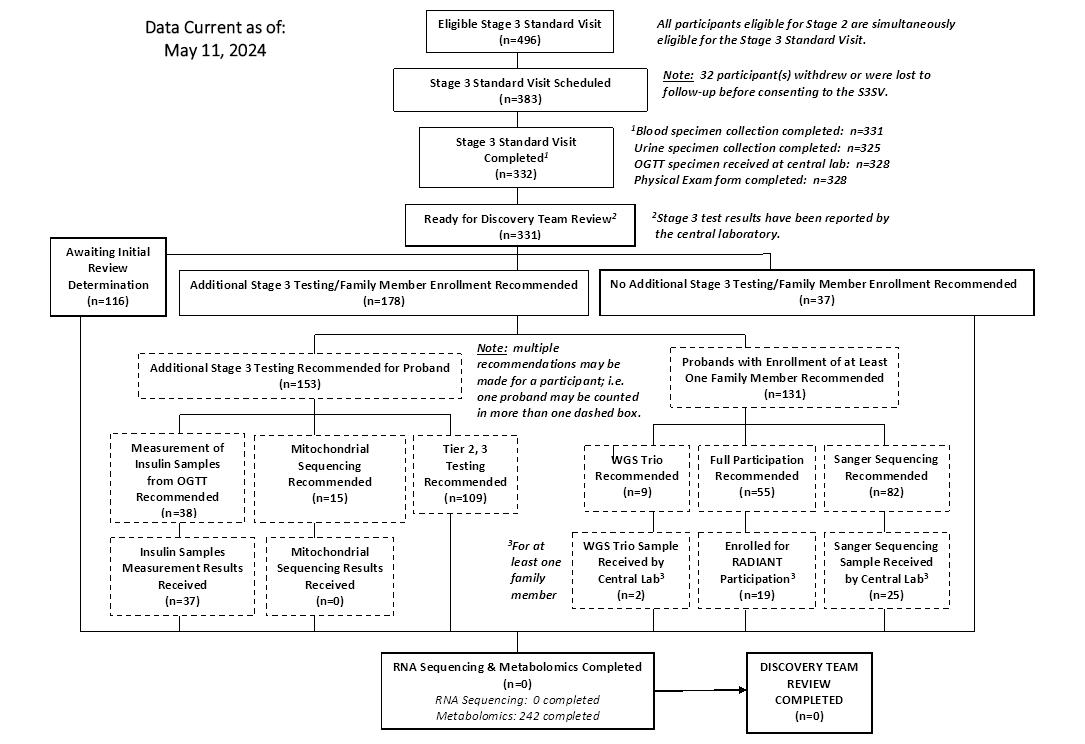
CONSORT Diagrams by RADIANT Study Stage
Documentation:
- RADIANT Clinical Metadata Collection Overview
- Overview of clinical metadata collected in RADIANT
- RADIANT Data Collection Summary
- Summary of form data and samples collected from RADIANT participants
- RADIANT Dataset Summary
- Description of RADIANT datasets produced from online questionnaires, clinical forms, and in-person visits
- RADIANT ‘Omics Data Overview
- Overview of ‘Omics analytes collected in RADIANT
Data Sharing
RADIANT has adopted policies and procedures in support of its commitment to sharing data with the scientific community while also protecting the privacy of participants.
RADIANT has established procedures to submit data to the NIDDK Central Repository in segments that reflect study progress. Access and availability of data submitted to the NIDDK Central Repository is determined by the NIDDK.
RADIANT has also established a policy for the release of RADIANT data not in the NIDDK Central Repository. Please refer to the RADIANT Policy on the Release of RADIANT Data and the Data and Materials Distribution Agreement (DMDA) for more information. Requests for access to data not in the NIDDK Central Repository may be submitted from the community of investigators currently working on RADIANT or from others who would like to collaborate with RADIANT in accordance with this policy and DMDA. Collaborators (i.e. researchers who are not RADIANT Study Investigators) who wish to access RADIANT study data need to team up with a RADIANT Study Investigator willing to sponsor their research as described in the DMDA.
To request access to RADIANT study data, please email the RADIANT DCC Team at
Ancillary Studies
Ancillary studies that complement the objectives of RADIANT and thereby enhance the value of RADIANT are encouraged. Researchers may submit ancillary study proposals to RADIANT for review in accordance with the RADIANT Ancillary Study Policy. Each ancillary study must be individually reviewed by the RADIANT Ancillary Studies Committee and approved by the RADIANT Steering Committee before its initiation.
RADIANT samples can be made available to researchers as part of an ancillary study following the submission and approval of a proposal to the RADIANT Ancillary Studies Committee. Please refer to the RADIANT Ancillary Study Policy for additional details. Please submit all proposals and related questions to the RADIANT DCC Team at
Additional Documentation
Please contact us with any questions or for more details.